Successful U.S. Market Entry by Lithuanian Medical Startup: Solution that Redefines Routine Clinical Practice is the Key
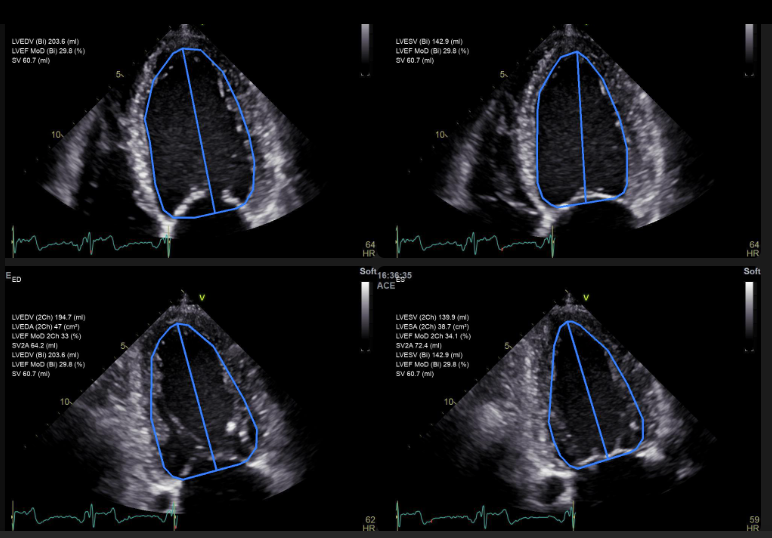
Lithuanian Medical Technology (MedTech) company “Ligence” received FDA 510(k) approval for artificial intelligence (AI) based cardiac ultrasound software “Ligence Heart”. The FDA is a health authority and regulatory agency that has the jurisdiction to approve the sale of medical devices to the U.S. market and guarantees the effectiveness and safety of health products and medical devices. It is one of the first FDA approvals of this type in the regions of Central, Northern and Eastern Europe for the automation of heart ultrasound research using artificial intelligence. With FDA approval, U.S. healthcare institutions will now be able to use the “Ligence Heart” for automated heart measurement analysis and structured reporting. In addition to ensuring measurement consistency and speeding up the ultrasound process — which takes a lot of time in many clinics due to manual measurements — this product is intended to decrease the quantity of manual measurements that doctors must complete.
What was the path of “Ligence” to this solid recognition in the U.S.? What are the FDA requirements? — we asked the founder and director of “Ligence”, Cardiologist Arnas Karužas. Our interlocutor is also a junior researcher at the Automation of Cardiovascular Investigation Laboratory of the Institute of Cardiology of the Lithuanian University of Health Sciences (LSMU), one of the coordinators of the project “SustAInLivWork” at LSMU.

A. Karužas: “The Food and Drug Agency of the United States of America (USA) certifies, evaluates various higher-risk products or, as in our case, software: whether such products can enter the U.S. market and be used in everyday clinical practice.
The path to this recognition was difficult. We had to present clinical evidence, a wide range of documentation to prove that the “Ligence Heart” software will be safe, effective and accurate enough to use it in everyday clinical practice with patients.
We had to demonstrate the unique benefits of “Ligence Heart” by demonstrating that the product was made to satisfy all specifications, including cyber security, accuracy, openness, replicability, and other requirements. Later, our software was tested in clinical centres in the USA with a variety of patient populations, and experts were included in these studies. Clinical studies have examined whether measurements based on Artificial Intelligence (AI) are sufficiently safe, accurate, or reach expert level and can be used in everyday clinical practice.
We have achieved this by proving that certain functionalities of “Ligence Heart” processes, measurements such as left ventricular ejection fraction — one of the key parameters for assessing the presence of heart failure — have passed the so-called threshold accuracy estimates.
All of these data, along with cybersecurity and other general requirements, were submitted to the FDA for evaluation — and then the evaluation process itself was carried out, additional questions were submitted, adjustments were made. Tests were also conducted separately with US users — cardiologists, ultrasound technicians: they tested the “Ligence Heart” and assessed whether they could use the software or not.
After all the clinical trials, the testing organisation made a decision. We are very pleased that we have become the first to receive such a certificate in this field in the whole region of Northern, Central and Eastern Europe. The FDA approval opens the door for our startup to fully enter the U.S. health sector market.”
What is this market?
“Due to its size, competitiveness, and readiness for innovation, as well as its vast population and significant investment in the health sector, the United States is one of the top markets for start-ups and businesses worldwide.
In the field of heart ultrasound, there are only a few companies around the world that directly work with the automation of the ultrasound examination of the heart using AI. Thus, we are the third in the 100-million-strong U.S. market to enter with an FDA certificate, and our “Ligence Heart” can be used in everyday clinical practice in the US.”
What trends do you observe, and what advice would you provide other healthcare start-ups?
“There are a lot of areas developing AI-based radiological solutions in the medical field at the moment, and I would encourage most of all not only general radiology, but rather specific areas of AI-based diagnostics for certain diseases, which are receiving a great deal of attention and investment worldwide. Another very promising area is medicine development processes, molecular development using AI.
The key is to find a solution that makes a real difference to everyday clinical practice and fits fully into it. It may seem straightforward, but it can be challenging to accomplish; frequently, it takes a long time to test a potential solution or innovation before discovering how it might fit into the clinical work process and have a big impact.
For there are many solutions that are good to have, but not essential, not those that could be called “game changers”. It is challenging to develop products that would transform daily practice, but if they are successful, both the developers and the consumers of the solution—patients and medical personnel—benefit greatly.”
What are your plans for the future?
“The “Ligence” team aims to make high-quality heart ultrasound examinations available not only in specialised cardiology centres, but also in other medical fields where ultrasound is
becoming more relevant. Automation reduces diagnostic variability and enables faster detection of changes in heart function.
The company plans to continue expansion in the US and European markets by expanding the functionality of the “Ligence Heart” product, seeking new 510(k) approvals and increasing the clinical value it provides.
The main priorities of the startup are now the European and US markets. Interesting and related markets are Canada, Japan, South Korea. Clinical trials have already been initiated in Australia.
We at the Lithuanian University of Health Sciences (LSMU) are happy to be able to keep up with the swift advancement of AI in the medical field. A recent research article published in the European Heart Journal Digital Health with an AI solution for the application of exercise echocardiography is the first article by Lithuanian authors in this high-profile journal.
LSMU has joined and together with other Lithuanian universities (KTU, Vilnius Tech and VMU) and international partners, is actively participating in a large project coordinated by Kaunas University of Technology called “Centre of Excellence in Artificial Intelligence for Sustainable Development” – “SustAInLivWork”
“The SustAInLivWork Centre of Excellence strengthens the research and innovation competences of the country’s scientists and researchers in the design and development of Artificial Intelligence (AI) solutions. The activities of the project provide opportunities to digitise the Lithuanian manufacturing, health, transport and energy sectors, promote the development of research, experimental development and innovation using AI-based solutions, and will significantly contribute to strengthening Lithuania’s international competitiveness.
New research works of doctoral students for the application of AI in medicine are already underway at LSMU. I am one of the first to defend my dissertation in this field soon – I am glad that more LSMU doctoral students and scientists are already working with various AI solutions.”
