Organoids: Scientific Innovation Redefining Disease Research, Diagnosis, and Treatment
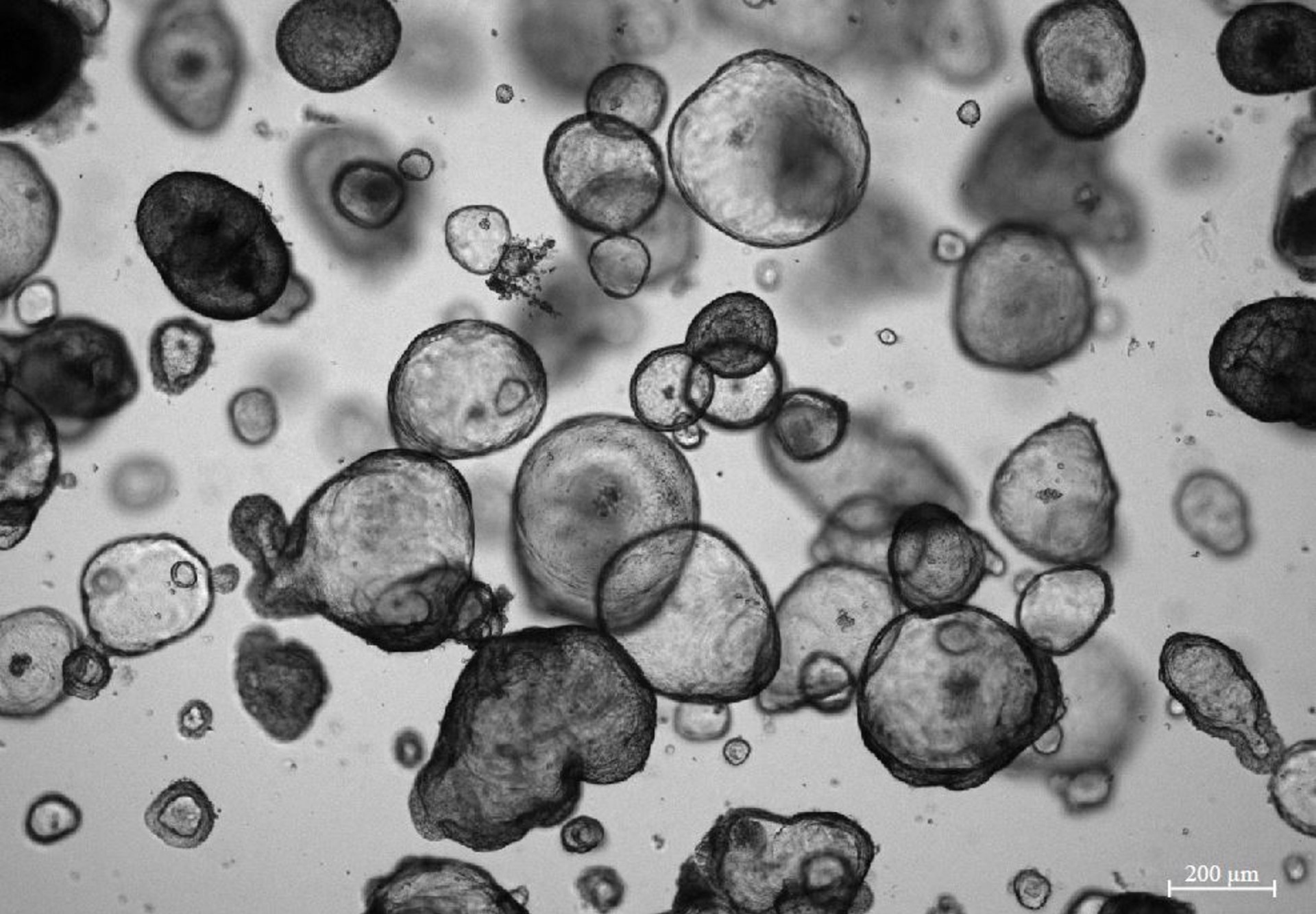
What are organoids? Why have they become one of the most innovative and cutting-edge models for disease research? How can organoid models of a gastrointestinal (GI) tract and their research help to expand our understanding of the pathogenesis of various complex diseases such as ulcerative colitis or Crohn’s disease? Professor Jurgita Skiecevičienė, Chief Researcher at the Clinical and Molecular Gastroenterology Laboratory of the Institute for Digestive Research at the Lithuanian University of Health Sciences (LSMU), has agreed to talk about the breakthrough in the use of organoid models.
Method of the Year
“Organoid research was voted Method of the Year six years ago by Nature, one of the most influential and highly cited scientific journals. This recognition reflects the importance and relevance of organoid research to the whole field of medicine and health sciences. This is exactly what has happened. In the last few years, organoids have been applied not only to fundamental research for the analysis of disease mechanisms and cell biology, but also to drug production, regenerative medicine, and many other fields,” said Prof. Skiecevičienė.
According to the Professor, organoids are mostly formed from so-called pluripotent stem cells. By applying a certain biological signal, it is possible to steer cell growth in the desired direction. Organoids can also be grown from already harvested medical tissues.
This model of cells of one or another organ, which has become a small in vitro, i.e. artificial, system, is already widely used. For example, the so-called “genetic scissors” (CRISPR-CAS technology) is already used to “correct” certain mutations and errors in the genome. This has already been proven worldwide to work in the case of cystic fibrosis mutations.
Organoids are also suitable for food analysis. This is exactly how the damage caused by excess sugar was revealed. When the study was later repeated on laboratory mice, most of those who received excess sugar died before the end of the experiment. In contrast, GI health improvements were observed in rodents fed a high-fibre diet.
“This means that the food we eat directly can affect cell metabolism and even stimulate repair and healing processes,” emphasised Prof. Skiecevičienė.
Science is Already Stepping Further
Research studies on organoids at LSMU has gone even further: co-cultures involving intestinal epithelial (organoid-derived) and immune cells are carried out, including work on co-cultures of bacteria and epithelial cells. These complex systems aim to reproduce cellular interactions in the body and to use them in basic research (e.g. host-bacteria interactions) or pre-clinical studies (e.g. drug trials).
Another extensive field of organoid research is bioengineering for regenerative transplantation. Scientists around the world are currently exploring the use of scaffolds to reconstruct damaged parts of organs: for example, by “reseeding” segments of the removed intestine with cultured healthy organoid epithelial cells, creating artificial assemblies of hydrogel systems, and by experimenting in vivo (i.e. in a live organism). In the in vivo experiments, a mouse has its epithelial layer removed, and then some of the human epithelial cells are added and observed for growth, adaptation, and structural maintenance.
Advanced Research Conducted at LSMU
For almost 20 years, a research team at the Clinical and Molecular Gastroenterology Laboratory of the LSMU Institute for Digestive Research, has been conducting advanced research on inflammatory bowel diseases such as ulcerative colitis and Crohn’s disease. Ulcerative colitis affects the large intestine, while Crohn’s disease affects the large and small intestine, and sometimes even the GI tract.
LSMU researchers are working intensively on genetic studies of these diseases. They have contributed to major whole-genome analysis studies, and have also contributed to exome studies to elucidate the genetic pathologies of Crohn’s disease and ulcerative colitis.
“We have carried out many microbiome studies, and have contributed to the finding that it is only the patient’s microbiome is altered, but even the microbiome of their first-degree relative is much less diverse than that of healthy people,” recalled Prof. Skiecevienė when asked to share with us one of the more interesting discoveries.
The Clinical and Molecular Gastroenterology Laboratory of the LSMU Institute for Digestive Research, conducts studies testing advanced biological therapeutics in in vitro systems.
Future projects will investigate metabolites from food that are broken down by bacteria. The researchers will look at how they can contribute to reducing inflammation. There are studies looking at the mechanisms of communication, or signalling, between bacteria and epithelial cells by co-culturing bacteria and epithelial cells.
This year, an EU-funded project was launched with partners from Christian-Albrecht University of Kiel (CAU), where a LSMU research team for digestive research is co-ordinating a large project MiGut Health (https://www.migut-health.eu), focusing on personalised medicine for inflammatory bowel diseases. The project has received funding from the Horizon Europe programme.
The project will aim to identify early diagnostic biomarkers, personalised prevention measures such as nutrition, lifestyle monitoring, and e-health tools.
The LSMU research team will intensively study the metabolites from food that are broken down by bacteria, to investigate how this relates to the status of inflammation and how to reduce it.
